When carbon monoxide (CO) is stored under high pressure in a steel gas cylinder, it reacts slowly to form iron pentacarbonyl (Fe(CO)5), a volatile impurity. To test this, we obtained a sample of spectroscopic-grade carbon monoxide from the local university’s chemistry department.
Using one of our SIFT-MS instruments, we analyzed the sample within seconds using full scan mode. We observed the characteristic isotopic fingerprint of iron pentacarbonyl for all three positive reagent ions (the negatively charged reagent ions are unreactive). H3O+ reacts with iron pentacarbonyl by the proton transfer mechanism, while NO+ and O2+ both react via the electron transfer mechanism. Using our LabSyft software, we determined a concentration of iron pentacarbonyl of 6 ppmV in what was ostensibly a high-purity carbon monoxide sample.
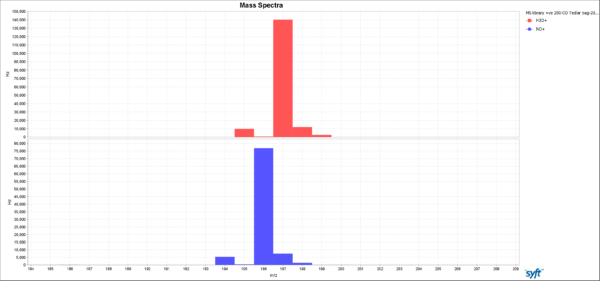
An interesting aside here is that the observed iron pentacarbonyl spectra provide an excellent illustration of just how soft the SIFT-MS chemical ionization is. The dominant peaks in the 70-eV electron impact ionization mass spectrum arise from FeCO+ (m/z 84) and Fe+ (m/z 56), whereas in the SIFT-MS spectra only the parent ion is observed from electron transfer ionization using NO+ and O2+ (Fe(CO)5+, m/z 196) and proton transfer ionization using H3O+ (Fe(CO)5.H+, m/z 197). In SIFT-MS, all CO ligands remain intact! This ultra-soft ionization is a key factor in the stable, reliable quantitation that is characteristic of SIFT-MS.
For rapid, stable trace gas analysis you can’t surpass SIFT-MS.
