Pharmaceutical
Residual Solvent Analysis
Rapid and sensitive detection of VOC residues in pharmaceuticals.
Rapid Residual Solvent Analysis
A wide variety of solvents are used during the manufacture of pharmaceuticals, but regulations strictly control permissible concentrations in finished products. SIFT-MS provides a very rapid and highly sensitive solution for the detection of VOC residues in pharmaceuticals. SIFT-MS provides rapid characterization of compounds that are not easily monitored by other technologies.
Benefits of SIFT-MS:
- Direct analysis through elimination of chromatographic separation, which is ideal for detection of residual solvents
- High selectivity via multiple rapidly switchable reagent ions
- SIFT-MS provides a very rapid and highly sensitive solution for the detection of VOC residues in pharmaceuticals and rapid characterization of compounds that are not easily monitored by other technologies.
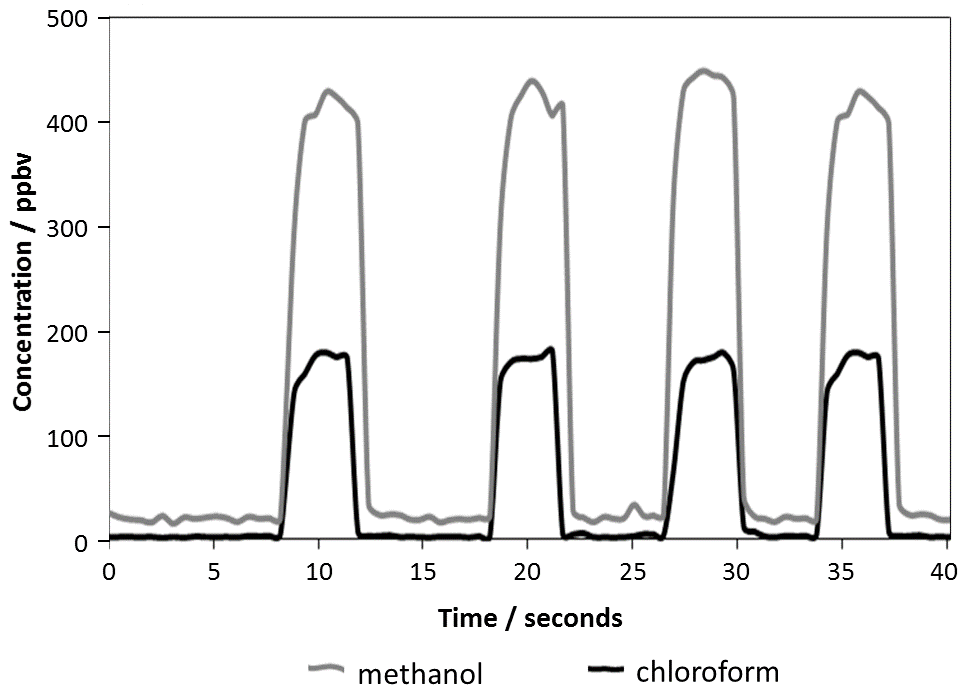
Rapid analysis for residual solvents using SIFT-MS.
Residual Solvent Analysis benefits
Comprehensive analysis, including inorganics and chromatographically challenging compounds
Flexible sample delivery options, including automation and area sampling
Intuitive software and fast method development
High sensitivity and wide linearity range
Automated high-throughput headspace analysis
Residual Solvent Analysis resources

Pharmaceutical Applications
SIFT-MS represents a major breakthrough for the pharmaceutical industry due to its ability to comprehensively analyze diverse VOCs and inorganic gas impurities with very high sample throughput. It quantifies VOCs directly in real-time to sub-part-per-billion (ppb) concentrations, so that product issues are detected earlier and resolved immediately, delivering economic benefits to all stakeholders.

Rapid Volatile Impurity Analysis in Pharmaceutical Products Using SIFT-MS
SIFT-MS quantifies volatile compounds that are chromatographically challenging such as formaldehyde, formic acid and ammonia. Direct, real-time analysis using SIFT-MS provides new opportunities across multiple pharma applications, including:
- Simple formaldehyde analysis
- Packaging screening, including residual monomer analysis
- Residual solvent analysis
- Cleaning validation
- Production facility air quality monitoring
- Bioreactor monitoring

Rapid, Simplified Residual Solvent and Volatile Impurity Analysis Using SIFT-MS
This webinar describes SIFT-MS applications in the pharmaceutical industry, including:
- Its use as an alternative procedure for USP <467> residual solvent analysis
- Rapid, quantitative analysis of leachable formaldehyde from polyoxymethylene polymer and PEG excipient
- High-throughput headspace screening for nitrosamine residues in drug products
- Fast turnaround, simplified analysis of ethylene oxide residues in detergents used for cleaning validation.

Pharma Method Validation - Formaldehyde
This application note demonstrates that by applying a strategy in accordance with International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Validation of Analytical Procedures: Text and Methodology Q2(R1) Guidelines (or “ICH Q2(R1)”) to direct analysis methods using SIFT-MS, successful validation is readily achieved – even for formaldehyde.

Direct MS Simplifies Analysis of Challenging Compounds
This webcast focuses on case studies that demonstrate simple analysis of chromatographically challenging compounds. Formaldehyde receives special attention, as it is important across a wide range of industries (from environmental to pharmaceutical testing).
Drying Endpoint Monitoring Using SIFT-MS For Enhanced Manufacturing Of Active Pharmaceutical Ingredients
Process analysis using SIFT-MS enables the drying process to be monitored past the drying end-point measurable using conventional weighing methods. This delivers greater efficiencies for production, and lowers risk of thermally damaging sensitive APIs.
SIFT-MS Selected Ion Flow Tube Mass Spectrometry
Learn about direct mass spectrometry by SIFT-MS which provides real-time, quantitative analysis of volatile compounds with trace-level sensitivity. There are also no requirements of chromatography, pre-concentration, or sample prep! SIFT-MS is easy to use and interpret data.

Simple, Rapid Analysis Of Ethylene Oxide In A Polysorbate 80 Excipient Using SIFT-MS
Quantitative ethylene oxide analysis in Polysorbate 80 excipient is greatly simplified using SIFT-MS, with a time to first test result that is eight-fold faster than the current compendial method and a daily sample throughput that is 9- to 14-fold higher.

The SIFT-MS Automation Series: Episode 6 SIFT-MS Automation Past, Present and Future
In this sixth and final episode, Dr Perkins reflects on the automation journey and shares his practical "hot tips" for a wide variety of automated analyses. The webinar (and series!) concludes with a discussion of how automated SIFT-MS is an excellent complementary technique to the conventional laboratory "workhorse" techniques: gas and liquid chromatography.

The SIFT-MS Automation Series: Episode 5 Continuous Headspace Analysis… and Beyond and R&D laboratories.
In this fifth episode, Dr Perkins describes the novel continuous headspace analysis (CHA) technique that he developed to measure stripping of volatiles. (Note: CHA is not to be confused with dynamic headspace analysis!). In addition, Mark describes simple, very high-throughput gas sample bag analysis and touches on other recent developments – including thermal desorption-SIFT-MS.

The SIFT-MS Automation Series: Episode 4 Calibration Approaches for Automated SIFT-MS
In this fourth episode, Dr Perkins describes how simply and rapidly traditional instrument calibration is achieved with the automated SIFT-MS configuration. Traditional calibration approaches have not featured regularly in the SIFT-MS literature due to the widespread use of reliable library-based quantitation. However, calibration is essential for contract testing laboratories, so it is addressed in detail and from a number of different angles in this webinar.

The SIFT-MS Automation Series, Episode 3: Advanced Headspace Methods with SIFT-MS
In this third episode, Dr Perkins describes the application of SIFT-MS to multiple headspace extraction (MHE). You will learn how to determine matrix-independent concentrations from MHE. Furthermore, Dr Perkins describes how the speed of SIFT-MS analysis can be leveraged to accelerate method development for both SIFT-MS and conventional methods – for example, through rapid determination of headspace equilibration times.

The SIFT-MS Automation Series, Episode 2: Accelerate Static Headspace Analysis with SIFT-MS
In this second episode, Dr Perkins provides a practical guide to automated static headspace (SH) analysis with SIFT-MS. You will learn how SH-SIFT-MS can address throughput challenges and simplify analysis of chromatographically challenging species.

The SIFT-MS Automation Series, Episode 1: Why Automate Analysis?
In this first episode, Dr Perkins answers the important question:
Why Automate Analysis?
This webinar introduces the benefits of automation and discusses how they can be applied to SIFT-MS, enabling straightforward adoption by the contract laboratory.
Simple, Rapid Analysis Of Formaldehyde Impurities In Gelucire Excipient Using SIFT-MS
SIFT-MS greatly simplifies formaldehyde detection and quantitation through direct, instantaneous, and sensitive (sub-ppbV) sample ionization, yielding sample throughputs of up to 250+ samples/day.
Simple Rapid Analysis of NDMA in a Recalled Valsartan Product Using SIFT-MS
Quantitative analysis of NDMA impurities in drug products is greatly simplified using SIFT-MS and has a three-fold throughput advantage (excluding sample prep benefits) over chromatographic methods.
A Consumer Safety Expert Shares His Experience with SIFT-MS
We asked David Light, CEO and Founder of Valisure, about his experience using SIFT-MS and how it impacted their dry shampoo safety study.

Rapid, Simplified Residual Solvent and Volatile Impurity Analysis Using SIFT-MS
SIFT-MS provides real-time, selective, and economic analysis of challenging volatile compounds, such as residual solvents, formaldehyde, dimethylamine, and NDMA without requiring derivatization or other special handling.
Syft Tracer: The Next Generation of Volatile Impurities Analysis for Enhanced Workflows
This app note introduces the next generation of SIFT-MS, Syft TracerTM, which launched at Pittcon 2023. It revolutionizes volatile impurities analysis workflows through unparalleled speed, performance stability, and reproducibility. Learn about how this innovation to real-time trace gas detection outpaces chromatography-based methods in the analysis of challenging analytes such as formaldehyde in a PEG excipient.
Head to Head Comparison of Class 2A And 2B Residual Solvents Analysis Using Sift-MS And GC-FID
This application note describes head-to-head comparison of GC-FID and SIFT-MS analyses of Class 2A and 2B residual solvents. The techniques perform similarly for linearity and repeatability, but SIFT-MS provides superior performance for accuracy and recovery. Furthermore, SIFT-MS provides greater than 11-fold increase in sample throughput and significantly reduces the time taken to report quantitative results (over six times faster for a full calibration set).
Recent developments and applications of selected ion flow tube mass spectrometry (SIFT‐MS)
SIFT‐MS is now recognized as the most versatile analytical technique for the identification and quantification of trace gases down to the parts‐per‐trillion by volume, pptv, range. This statement is supported by the wide reach of its applications, from real‐time analysis, obviating sample collection of very humid exhaled breath, to its adoption in industrial scenarios for air quality monitoring. This review touches on the recent extensions to the underpinning ion chemistry kinetics library and the alternative challenge of using nitrogen carrier gas instead of helium.

The Latest Innovation of Real-Time, High-Throughput Volatile Impurities Analysis by SIFT-MS
Join us for this webinar to learn about Syft Tracer, the latest advancement of real-time, trace gas analysis by SIFT-MS which launched at Pittcon 2023. Hear how the recent product innovations unlock analytical bottlenecks and enable faster decisions to be made in critical process steps.
Even Faster Quantitation Of Formaldehyde In Gelucire Excipient
This application note describes how the efficiency of a MHE workflow can be significantly improved for MHE-SIFT-MS due to the stability of the technique. Formaldehyde impurity is analyzed easily and quantitatively in Gelucire excipient with this improved approach. The time-to-result is reduced to 85 minutes for this system – six-fold faster than the conventional MHE-SIFT-MS approach. Quantitative analysis is achieved at the throughput of 220 samples/day.
Revolutionary Productivity For Volatile Residue and Impurity Analysis
This application note describes a scenario in which Syft TracerTM analyzed the same amount of samples in 9 hours that it took 5 chromatographic systems 24 hours to complete and still had capacity to spare. Next-gen SIFT-MS provides rapid, chromatography-free workflows which revolutionize volatile compound analysis.
Application Brief: Revolutionary Workflows for Volatile Impurities Analysis
This application brief summarizes a scenario relevant to contract research organization (CRO) and contract drug manufacturing organization (CDMO) laboratories where multiple volatile impurity methods need to be conducted in short runs. Five analyses are considered that can be handled by one Syft Tracer but require multiple legacy, chromatography instruments. For these analyses, SIFT-MS reports the first quantitative results 2- to 12-fold faster, and has sample throughputs 3- to 17-fold higher than the conventional procedures.
Headspace-SIFT-MS: Flexibility that Revolutionizes Workflows for Diverse Samples
The characteristic flexibility, stability, high throughput, and fast time to data of the Syft Tracer next-gen SIFT-MS instrument apply across multiple headspace approaches for diverse matrices. This application note briefly summarizes the use of (1) dissolution, (2) multiple headspace extraction (MHE), and (3) the method of standard additions, then provides a guide for identifying the appropriate headspace approach for various matrices.
Extending SIFT-MS Residual Solvent and Volatile Impurity Analysis to Water-Insoluble Articles
SIFT-MS can analyze residual solvents and other volatile impurities in water-insoluble articles using a two-step process: dissolution in compatible solvent, then dilution in water. This application note describes a systematic evaluation of the compatibility of six organic solvents with headspace-SIFT-MS analysis following this two-step process. Quantitative headspace analysis is achieved at 12 samples/hr, providing significant productivity improvements compared with chromatographic approaches.

Revolutionizing Workflows for Residual Solvents and Volatile Impurities Analyses by SIFT-MS
This webinar demonstrates how the new, automated Syft TracerTM SIFT-MS platform provides a comprehensive solution to workflow challenges and can replace multiple chromatographic systems. Learn about how combining SIFT-MS with automation provides a very flexible and high throughput solution for screening volatile impurities in pharmaceutical and consumer safety applications, revolutionizing workflows.
Rapid Sensory Analysis of Paper Packaging Using SIFT-MS
This application note demonstrates that SIFT-MS effectively classifies paper samples by odor intensity rating and odor descriptor, in addition to distinguishing paper composition and mill of origin. When integrated with autosamplers, SIFT-MS provides throughputs of over 220 samples per day, supporting significantly enhanced quality assurance compared to conventional human sensory panels and gas chromatography-based analyses.
Pharmaceutical Residual Solvent Analysis: A Comparison of GC-FID and SIFT-MS Performance
This study expands upon the previous work by conducting a head-to-head comparison of GC-flame ionization detection (GC-FID) and SIFT-MS procedures. SIFT-MS analyzed samples over 11-fold faster than GC-FID, increasing daily sample throughput and reducing the time taken to determine the result. The results suggests that residual solvent analysis using SIFT-MS may support workflow improvements for pharmaceutical manufacturers.
Revolutionizing Volatile Impurities Analysis Through Next Gen SIFT-MS
This E-book describes how next gen SIFT-MS enables rapid, continuous screening of toxic volatile impurities in pharmaceutical and consumer products. This advancement to SIFT-MS delivers trace-level detection sensitivity, unparalleled performance stability, superior selectivity, and highly reproducible, quantitative data. Never miss a contamination event again.
Improved MHE-SIFT-MS Workflows - Concentration Independent MHE Calibration
This application note investigates concentration dependence of MHE calibration in sample matrix. Across the full range of analytes investigated in this study, MHE calibration holds for at least one order of magnitude change in sample concentration. For analytes in the C7–C9 range, the MHE calibration applies over two orders of magnitude analyte concentration. These results mean that the MHE workflow can be applied to a wider range of samples in the matrix, further reducing calibration demand.
Real-Time Analysis of Volatile Emissions from Hot-Melt Extrusion using Untargeted SIFT-MS
SIFT-MS provides unique capabilities that enable formulators to optimize the hot melt extrusion processes by minimizing polymer, API, and other excipient degradation. SIFT-MS provides real-time feedback though online, ultra-trace, gas-phase analysis of the small molecules emitted when the mixture is extruded, benefiting process development and optimization, as well as continuous process monitoring and control.
Residual Solvent Analysis: Optimization for DMI Solvent
DMI is a very promising non-aqueous solvent because it can be used even without subsequent dilution in water. Using a subset of residual solvents, this study confirms that moderately polar volatiles yield headspace responses that are independent of the volume of sample used. This means that cost and environmental impact can be reduced. Furthermore, with SIFT-MS this optimization can be carried out very rapidly due to direct sample analysis and high sample throughput.
Rapid Analysis of Residual Solvents and Volatile Impurities for High-Throughput CDMO Workflows
In this webinar, we introduce our new compliant real-time mass spectrometer solution, Syft Tracer Pharm11. A single instrument is capable of running up to 220 samples per day, and multiple methods and analyses can be performed in sequence. The direct headspace-SIFT-MS instrument is chromatography-free, and therefore the need for multiple columns and instrument downtime between methods does not exist. We will highlight several common CDMO tests that have been performed and validated using headspace SIFT-MS, including residual solvents, ethylene oxide, nitrosamines and other extractables & leachables.
Faster Quantitative Analysis of Volatile Impurities Using MHE-SIFT-MS
In this application note, enhanced MHE workflows are demonstrated using styrene, formaldehyde, and NDMA analyses in polystyrene polymer, Gelucire excipient, and ranitidine drug products, respectively. Reduced calibration frequency in routine analysis enables significant workflow benefits to be realized, including four-fold faster time to first result for quantitative analysis of condensed-phase samples. Over 220 samples per day can be analyzed quantitatively for diverse volatile impurities using the enhanced MHE-SIFT-MS workflow.

SIFT-MS: Real-Time Volatiles Analysis for Continuous Manufacturing of Pharmaceuticals
Watch this on-demand webinar featuring Mark Perkins (Element), Professor Chris Price (CMAC, University of Strathclyde) and Aaron Smith (CMAC, University of Strathclyde) to learn how SIFT-MS provides real-time analysis of volatile impurities when determining solvent drying end points, volatile degradation products, or volatile impurities during hot melt extrusion.
Accelerating residual solvents analysis in 21 CFR Part 11 compliant settings through real-time mass spectrometry
SIFT-MS is a direct, real-time mass spectrometry (MS) technique which offers revolutionary volatile compound analysis capabilities to Pharma and CDMO labs due to its fast time to data, time efficient workflows, analytical flexibility, and ease of use. It expedites analytical workflows, such as residual solvents analysis, by generating faster results than traditional methods. Syft Tracer Pharm11 is a SIFT-MS-based solution that includes SyftAuditTracer software designed for 21 CFR Part 11 compliant environments. This app note describes how volatile impurities can be characterized in real-time including nitrosamines, ethylene oxide, and residual solvents.
Supporting 21 CFR Part 11 Regulated Workflows with Next Gen SIFT-MS
This white paper describes how SyftAuditTracer software supports compliance with 21 CFR Part 11 regulations in pharma and CDMO environments. SyftAuditTracer is part of the Syft Tracer Pharm11 bundle solution for high-throughput, compliant workflows.
